Partnership Opportunities
Forge Strategic Connections at the Medical Device Software Development Summit
The Medical Device Software Development Summit is where high-level, game-changing partnerships are made. Tailored with distinct technical and regulatory tracks, this summit offers a focused platform for senior decision-makers from software development, quality assurance, and regulatory teams in the medical device industry. These professionals are actively seeking solutions that address their most urgent challenges - whether in AI, cybersecurity, compliance, or development efficiency.
In this niche, results-driven environment, our partners engage directly with a pre-qualified audience of leaders and innovators who are shaping the future of medical device software. With a clear focus on driving forward healthcare technologies, the summit ensures every connection made is meaningful and focused on mutual success.
Based on our research, the industry is actively searching for the following services:

Software Development Platforms: As companies race to bring devices to market, they rely on robust development platforms to enhance efficiency, scalability, and integration capabilities.

Cybersecurity Software: With increasing threats of data breaches and device vulnerabilities, developers need cutting-edge security solutions to protect patient safety and maintain regulatory compliance.

Regulatory Consultants: Navigating the ever-changing landscape of global medical device regulations requires expert guidance to ensure compliance, accelerate approvals, and mitigate risks.
Testing & Quality Management: From verification and validation to risk assessment and post-market surveillance, ensuring product reliability and regulatory approval is a top industry priority.
Past Sponsors Include:

Let's Build a Strategic Partnership
We offer tailored sponsorship packages to align with your goals—whether through thought leadership sessions, one-on-one meetings, exhibit booths, or exclusive networking opportunities. Connect with us today to explore how you can maximize your impact at the summit.
Sponsor Testimonials

“Great quality of presentations, domain knowledge of the speakers and participants, excellent networking opportunities” Principle, Digital Health & Program Manager, Analog Devices

“Quality speakers. Quality audience. Good opportunities to connect with interesting people” Chief Solutions Officer, Orthogonal

“Very relevant topics and discussions with a broad range of companies represented” Director, Regulatory Affairs & Quality, Cumulus Neuroscience
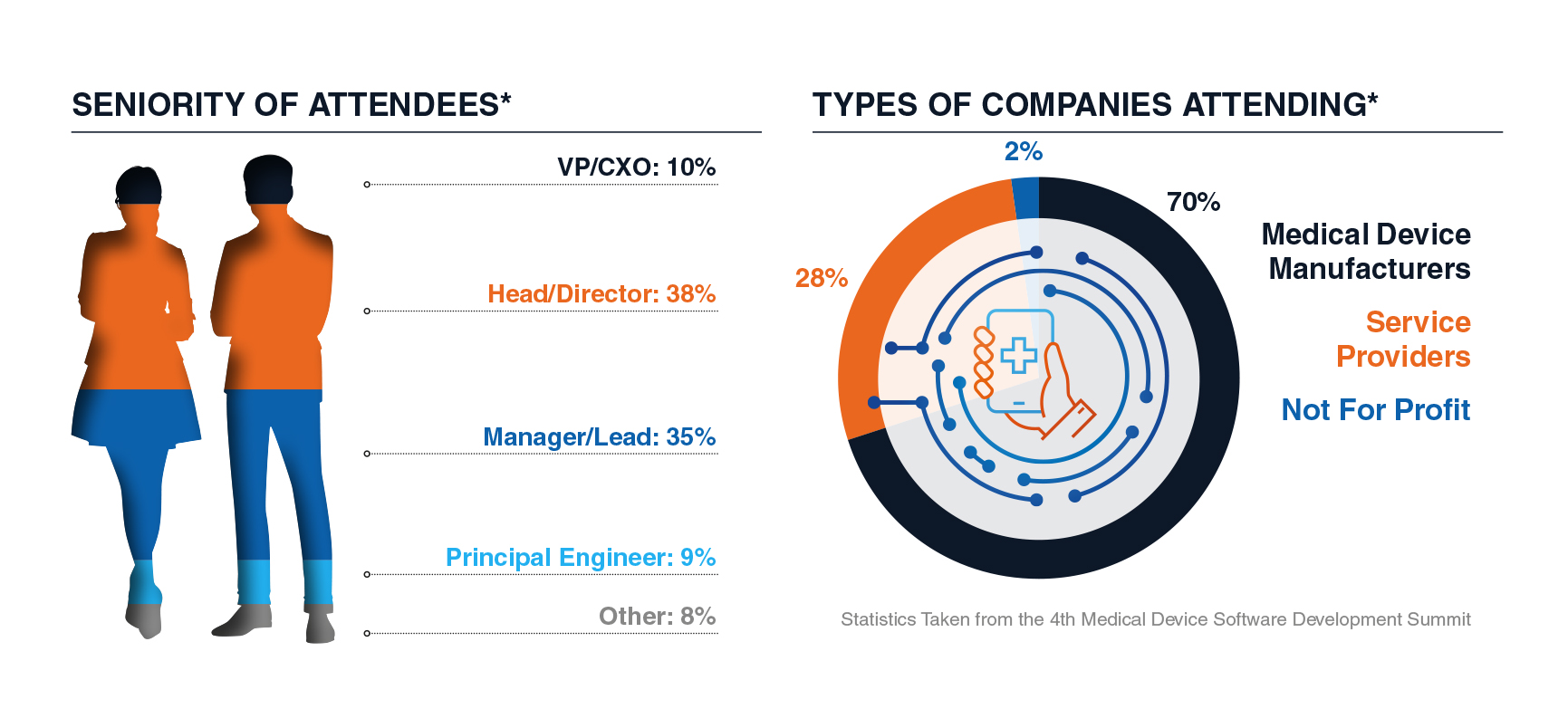
Who Our 2025 Partners Met:
Let's Build a Strategic Partnership
Companies We’ve Welcomed in the Past:

