About the 2025 event
What You Missed at the 5th Medical Device Software Development Summit:
With the FDA undergoing significant restructuring—including key layoffs in medical technology regulation—uncertainty is growing around how evolving policies will impact software development for medical devices. At the same time, increasing scrutiny on cybersecurity, AI validation, and post-market surveillance is pushing companies to rethink their compliance strategies.
The 5th Medical Device Software Development Summit addressed the ways to get ahead of these rapid changes, ensuring your organization is prepared for the regulatory shifts and technological advancements shaping the future of medical device software
- Address the Growing Cybersecurity Threat – The industry is seeing heightened scrutiny over software vulnerabilities—discover how to future-proof your devices against cyber risks while maintaining performance.
- AI & Next-Gen Tech in the Spotlight – With increased FDA oversight on AI-powered diagnostics and real-time software systems, learn how to balance innovation with regulatory compliance.
- Adapt to a Competitive & Rapidly Changing Market – As new entrants push innovation forward and compliance hurdles become more complex, establish your company as a leader by staying ahead of industry shifts.
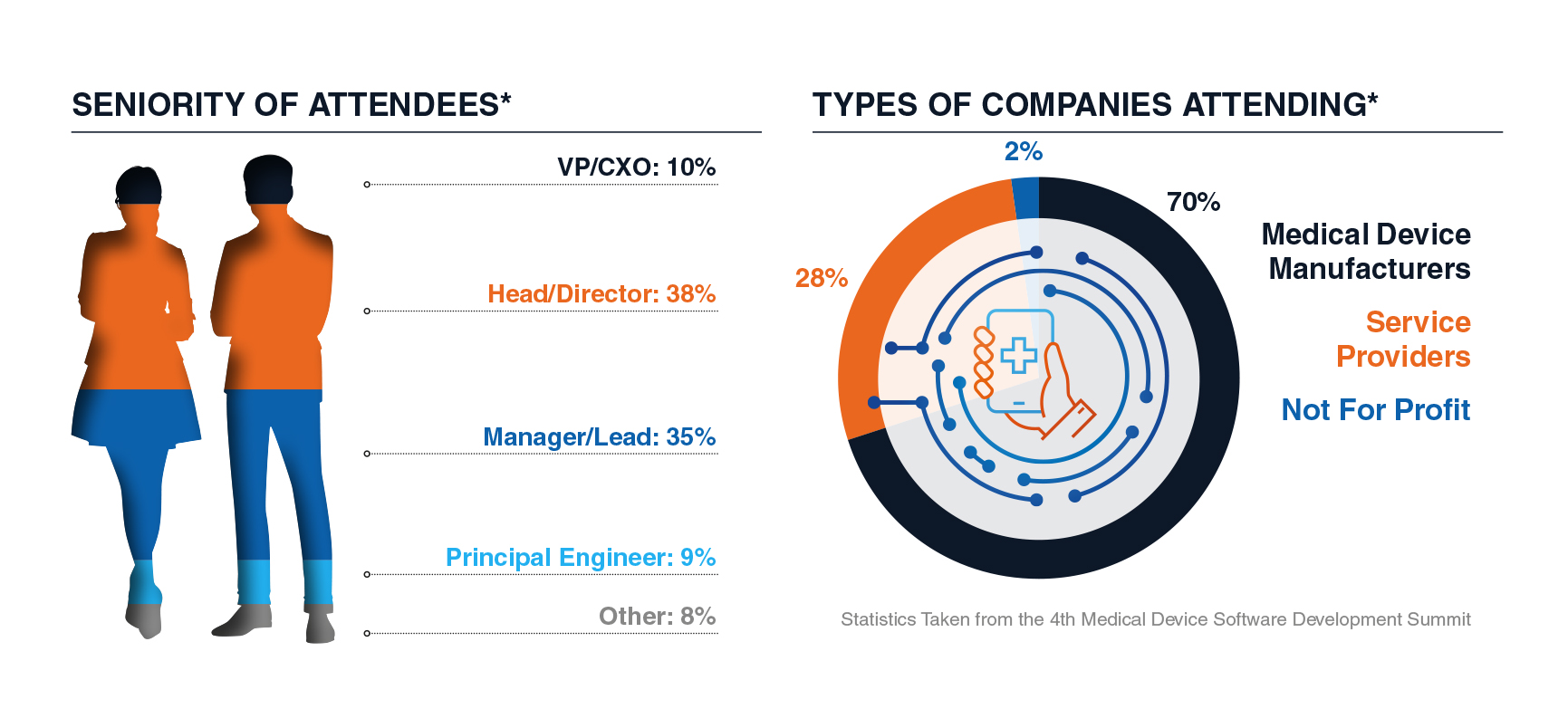
Who Attendees Met:
Past Participants included:


