Join Industry Experts Shaping the Future of Regulated Medical Software
Medical device software teams are navigating regulatory uncertainty, rising cybersecurity expectations, and accelerating AI innovation, all while being pushed to deliver faster, safer, and with stronger evidence.
The 6th Medical Device Software Development Summit brings together 50+ senior leaders from the likes of Medtronic, Philips, Boston Scientific and Siemens Healthineers who are actively seeking solutions to modernize development, strengthen compliance, and scale securely.
By partnering, you’ll gain direct access to a highly targeted audience of decision-makers with live projects and defined budgets, not passive attendees. Walk away with qualified leads, strategic relationships, brand positioning alongside industry leaders, and direct insight into the challenges shaping purchasing decisions in 2026. This is your opportunity to be seen as a trusted partner enabling compliant innovation in medical device software.

What to Expect as a Partner?
Gain Direct Market Insight
Hear unfiltered challenges from practitioners navigating FDA guidance updates, AI governance, DevSecOps implementation, and global regulatory harmonization, helping you refine your value proposition and go-to-market strategy.
Meet High-Value Prospects
Connect face-to-face with 50+ senior leaders across Software, Quality, Regulatory, Cybersecurity, and Product functions who are actively tackling AI validation, cybersecurity compliance, and regulatory submission challenges.
Strengthen Brand Credibility
Position your organisation alongside respected MedTech leaders through curated branding across the agenda, brochure, and on-site materials within a content-led, practitioner-focused environment.
Influence Strategic Thinking Early
Be part of the conversations shaping how companies approach validation, documentation, DevSecOps, and global compliance, allowing you to inform solution direction before formal vendor selection begins.
Understand Buying Drivers First-Hand
Hear directly from software, quality, and regulatory leaders about current operational pressures, internal approval hurdles, and upcoming investment priorities, equipping you with sharper market intelligence for 2026 planning.
Key Interest Areas
Our attendees from leading medical device manufacturers and digital health innovators are actively seeking service and solution providers with capabilities in the below areas, but not limited to:
Software Development Platforms
Cybersecurity Software
Regulatory Consultants
Manufacturing, Testing & Quality Management
Hear What Our Past Sponsors Have to Say

I liked the smaller intimate format. I was pleasantly surprised by the quality of AI discussion as well
Business Development, Runsafe Security

The content and speakers were very strong, relevant, and educational. Also, they liked the speed networking
Director Field Marketing, Cielo
Audience Composition
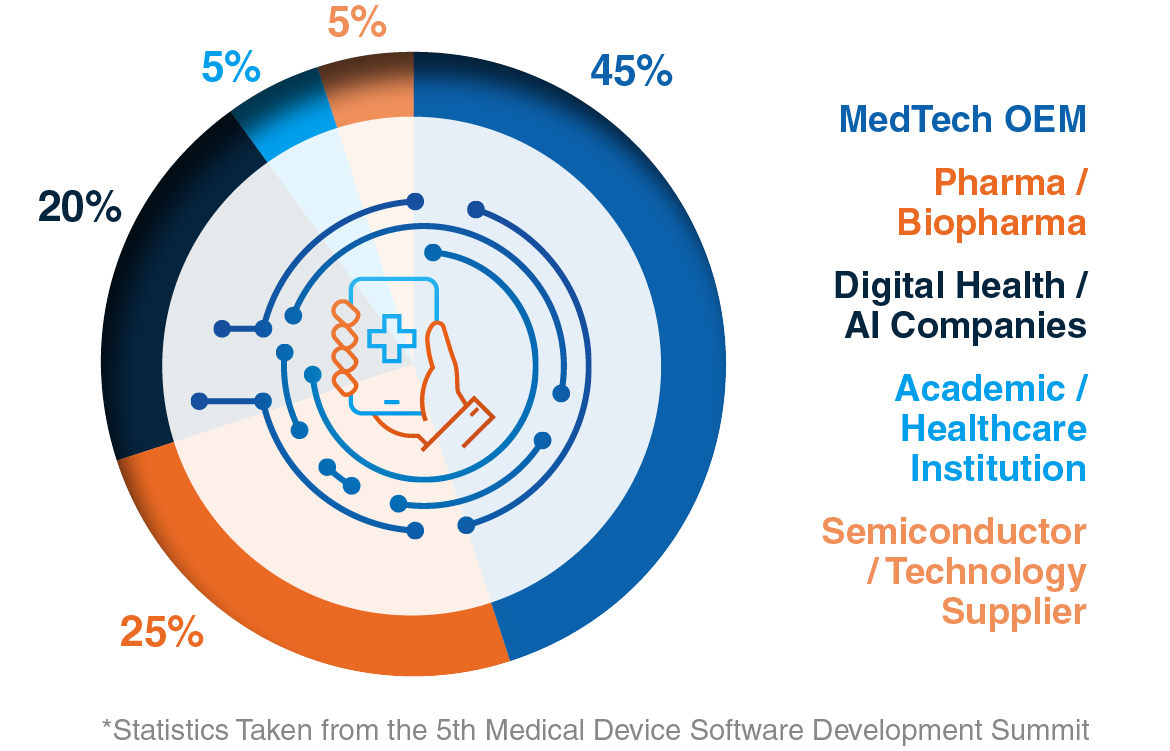
Company Type
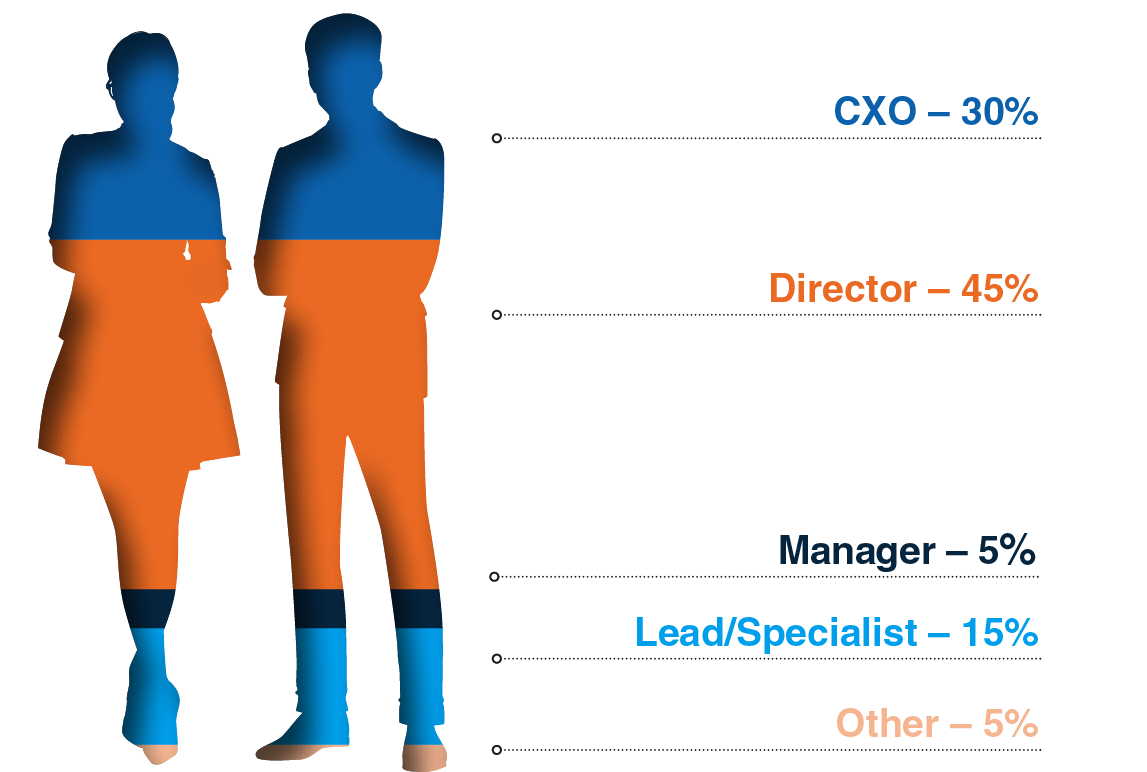
Attendee Seniority
Attending Companies Include








Available Inventory
We have a range of inventory from standard booth and speaking slots to more bespoke deliverable such as badges, exclusive dinners and tours to help elevate your brand.








Get in Touch
Take advantage of our bespoke sponsorship opportunities to achieve your commercial goals. Email us if you would like to get involved and discuss a bespoke package suited to your needs.

Adam Grosz
Business Development Manager



